Key Market Insights
- Presently, more than 70 companies claim to have the required expertise to offer various services for the synthesis and manufacturing of mRNAs across different scales of operations, worldwide
- In pursuit of building a competitive edge, service providers are actively upgrading their existing capabilities to enhance their respective portfolios and comply with the evolving industry benchmarks
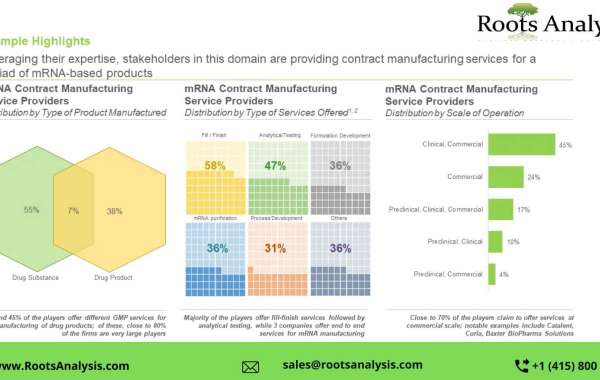
- Leveraging their expertise, stakeholders in this domain are providing contract manufacturing services for a myriad of mRNA-based products
- Presently, more than 95 kits are available in the market for the synthesis of research grade mRNAs used for various applications with yield range up to 50,000 µgs
- Close to 50% of mRNA synthesis kits contain all the components, including enzyme mix, buffers and other reagents, required for the synthesis of varying quantity of modified mRNAs at affordable prices
- mRNA synthesis kit providers are steadily expanding their capabilities in order to enhance their existing capabilities and augment their respective kit portfolios
- A case study of considerable increase in the number of clinical trials registered for mRNA-based therapeutics / vaccines shows growing demand for such therapeutic / preventive interventions
- The growing interest is evident from the rise in partnership activity; in fact, the maximum number of collaborations related to mRNA-based drug manufacturing were inked in 2020 and 2021
- Close to 35 mRNA-based therapeutic / vaccine developers across the world are anticipated to forge strategic alliances with mRNA synthesis and mRNA manufacturing service providers, to further augment their drug portfolio
- In order to tap the lucrative opportunity in this rapidly growing market, big pharma players have undertaken several initiatives, including strengthening product portfolio, agreements, and investments
- The market’s evolution is likely to be driven by the need for novel mRNA therapeutics; we expect the future opportunity to be well distributed across various types of product, therapeutic areas, and key geographical regions
- PREFACE
1.1. Scope of the Report
1.2. Market Segmentations
1.3. Research Methodology
1.4. Key Questions Answered
1.5. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. An Overview of mRNA
3.2. Structure of mRNA
3.3. Evolution of mRNA Vaccines
3.4. mRNA Manufacturing Process
3.5. Applications of Chemically / in vitro Synthesized mRNA
3.6. Challenges Associated with mRNA Synthesis
3.7. Commonly Outsourced Manufacturing Operations
3.8. Advantages of Outsourcing Manufacturing Operations
- MARKET LANDSCAPE: mRNA CUSTOM SYNTHESIS SERVICE PROVIDERS
4.1. mRNA Custom Synthesis Service Providers: Overall Market Landscape
- mRNA CUSTOM SYNTHESIS SERVICE PROVIDERS: COMPANY COMPETITIVENESS ANALYSIS
5.1. Key Parameters and Scores
5.2. Methodology
5.3. Competitiveness Analysis: Small Companies
5.4. Competitiveness Analysis: Mid-Sized Companies
5.5. Competitiveness Analysis: Large Companies
- MARKET LANDSCAPE: mRNA CONTRACT MANUFACTURING SERVICE PROVIDERS
6.1. mRNA Contract Manufacturing Service Providers: Overall Market Landscape
- mRNA CONTRACT MANUFACTURING SERVICE PROVIDERS: COMPANY COMPETITIVENESS ANALYSIS
7.1. Methodology
7.2. Company Competitiveness Analysis: mRNA Contract Manufacturing Service Providers based in North America
7.3. Company Competitiveness Analysis: mRNA Contract Manufacturing Service Providers based in Europe
7.4. Company Competitiveness Analysis: mRNA Contract Manufacturing Service Providers based in Asia-Pacific
- MARKET LANDSCAPE: mRNA SYNTHESIS KIT PROVIDERS
8.1. mRNA Synthesis Kits: Overall Market Landscape
8.2. mRNA Synthesis Kits: Developer Landscape
- mRNA SYNTHESIS KITS: PRODUCT COMPETITIVENESS ANALYSIS
9.1. Methodology
9.2. Product Competitiveness Analysis: mRNA Synthesis Kits
- COMPANY PROFILES: mRNA SYNTHESIS AND MANUFACTURING SERVICE PROVIDERS
10.1. Aldevron
10.2. Biomay
10.3. bioSYNTHESIS
10.4. eTheRNA
10.5. Eurogentec
10.6. TriLink BioTechnologies
- COMPANY PROFILES: mRNA SYNTHESIS KIT PROVIDERS
11.1. APExBIO
11.2. CELLSCRIPT
11.3. Jena Biosciences
11.4. New England Biolabs
11.5. Thermo Fisher Scientific
- CLINICAL TRIAL ANALYSIS
12.1. Methodology
12.2. mRNA-based Therapeutics / Vaccines: Clinical Trial Analysis
- PARTNERSHIP AND COLLABORATIONS
13.1. Partnership Models
13.2. mRNA Synthesis and Manufacturing: Partnerships and Collaborations
- LIKELY PARTNERS ANALYSIS
14.1. Methodology
14.2. Likely Partners based in North America
14.3. Likely Partners based in Europe
14.4. Likely Partners based in Asia Pacific and Rest of the World
- BIG PHARMA INITIATIVES
15.1. Methodology
15.2. Big Pharma Players: List of mRNA-based Therapeutics / Vaccines Focused Initiatives
15.3. Competitive Benchmarking of Big Pharma Players
- MARKET FORECAST
16.1. Chapter Overview
16.2. Key Assumptions and Forecast Methodology
16.3. Global mRNA Synthesis and Manufacturing Market, 2022-2035
- EXECUTIVE INSIGHTS
- APPENDIX 1: TABULATED DATA
- APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/mrna-synthesis-and-manufacturing-market.html
You may also be interested in the following titles:
Novel Cell Cytometers: Need of the Hour
Viral Clearance and Testing Services Market
Roots Analysis Consulting - the preferred research partner for global firms
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415